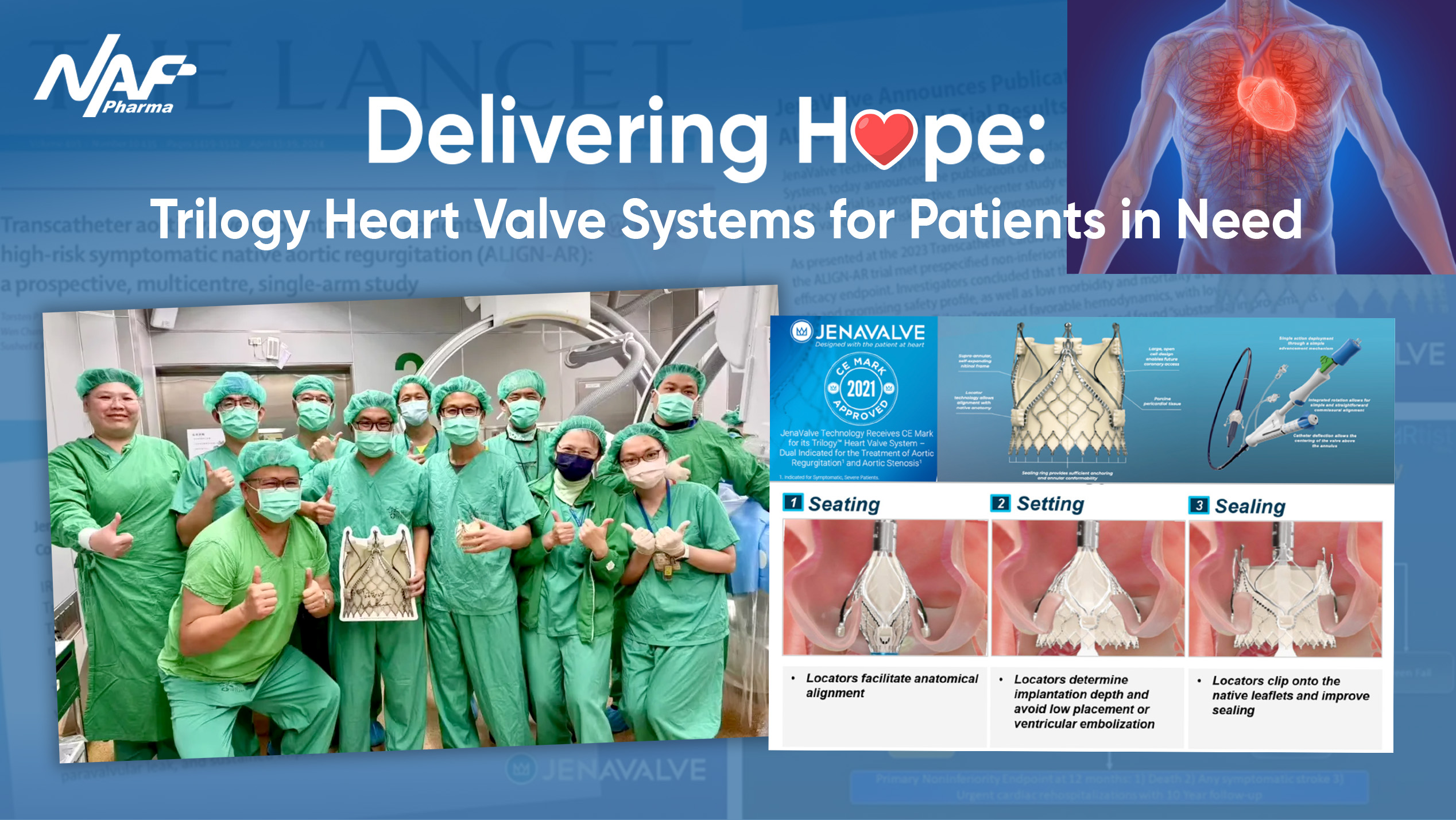

On March 3, 2025, Taiwan witnessed the first two implantations of the "Trilogy Heart Valve System" at National Taiwan University Hospital, the world’s first TAVR (Transcatheter Aortic Valve Replacement) solution approved for isolated AR(Aortic Regurgitation).
Averaging just 1.5 hours per case, under 30 minutes from sheath-in to sheath-out, Trilogy Heart Valve System's four key components (Trilogy Heart Valve, Trilogy Delivery Catheter, Trilogy Loading Tools, and Trilogy Sheath/Dilator) worked in sync to deliver life-saving results.
None of this would’ve been possible without NAF’s logistics expertise.
✅ Biological Preservation: We maintain hydration and strict temperature control for the valve’s porcine tissue.
✅ Structural Safety: Specialized packaging shields the nitinol frame and the Trilogy system from transit damage.
✅ Sterility Guaranteed: NAF's robust packaging withstands shipping stress and keeps components contamination-free.
With its CE mark in Europe secured and FDA approval expected by late 2025, the Trilogy system is anticipated to significantly boost the global TAVR market. For those once deemed untreatable, hope has arrived—and NAF is proud to have delivered it.
Interested in working with us?
Get a quote for free and one of our pharma logistics experts will be in touch to discuss your specific needs.









